Chemistry Experiments and important reactions —— Electrical chemistry
Index
- Chromatography
- Common experiments operations
- Tests for ions and gases
- Extraction of metals
- Electrical chemistry
- Haber process
- Contact process
- Carbon cycle
- Purification of water
- Addition reaction
- Substituion reaction
- Methods to produce ethanol
- Cracking of organic compounds
- Fractional distillation of petrol
- Polymerization
Electrical chemistry
Eletrical cell
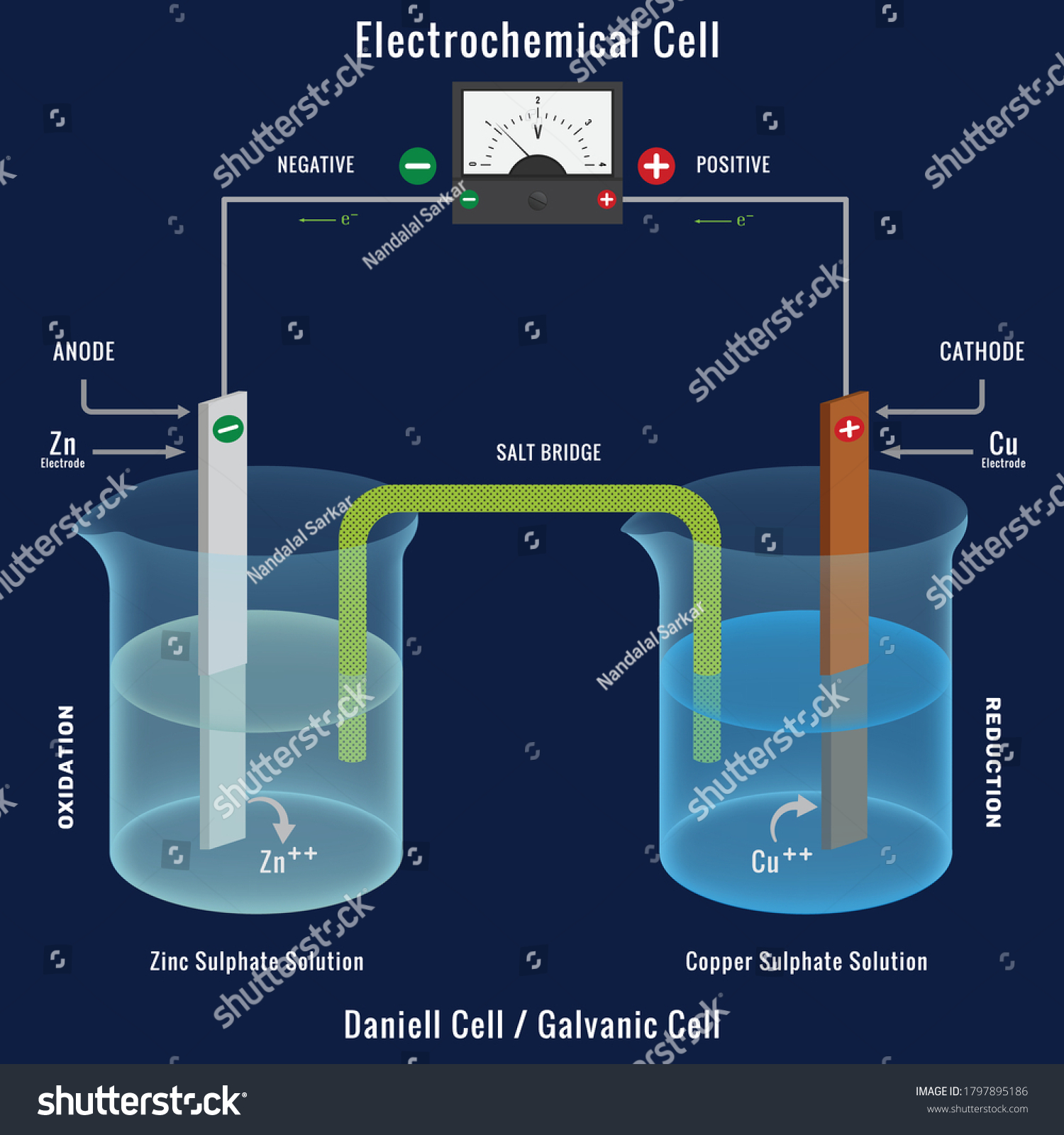
- In electrical cell, anode is the negative electrode while cathode is the positive electrode
- The electrons transfer from anode to cathode
- The more reactive metal is usually anode
- Exothermic redox reaction must occur in electrical cell
Electrolysis
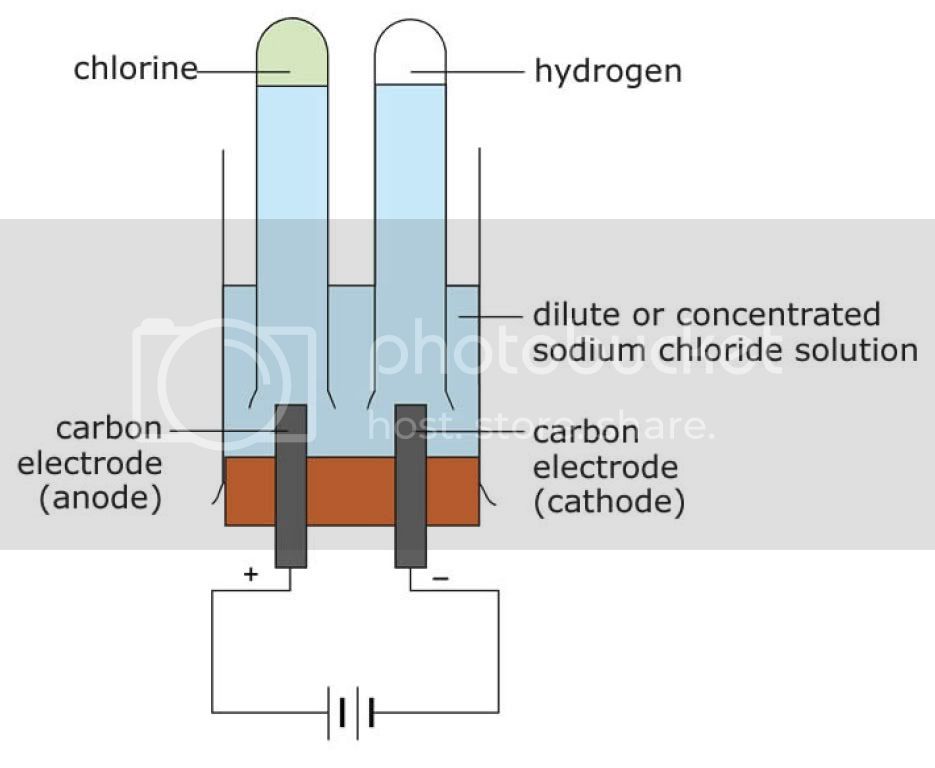
- Usually, in electrolysis, anions will be attracted to anode and cations will be attracted to cathode
- The more important thing is the discharging series for cations and anions
- Cations(Cathode)
- Ag > Hg > Fe(III) > H > Pb > Sn > Fe(II) > Zn > H(water) > Al >Mg > Ca > Na > K
- Anions(Anode)
- S > I > Br > Cl > OH > -ate > F
- Cations(Cathode)
- Also, concentration of ions can also influence its product
Electroplating / Refining metals

- Electroplating is used to coat metals like iron, which can improve its ability to prevent from corrosion
- There is not a full reaction happens when elctroplating or refining metals
- There are only eletrons transferring in the circuit
本博客所有文章除特别声明外,均采用 CC BY-SA 4.0 协议 ,转载请注明出处!