Chemistry Experiments and important reactions —— Extraction of meatals
Index
- Chromatography
- Common experiments operations
- Tests for ions and gases
- Extraction of metals
- Electrical chemistry
- Haber process
- Contact process
- Carbon cycle
- Purification of water
- Addition reaction
- Substituion reaction
- Methods to produce ethanol
- Cracking of organic compounds
- Fractional distillation of petrol
- Polymerization
Extraction of metals
Extraction of iron
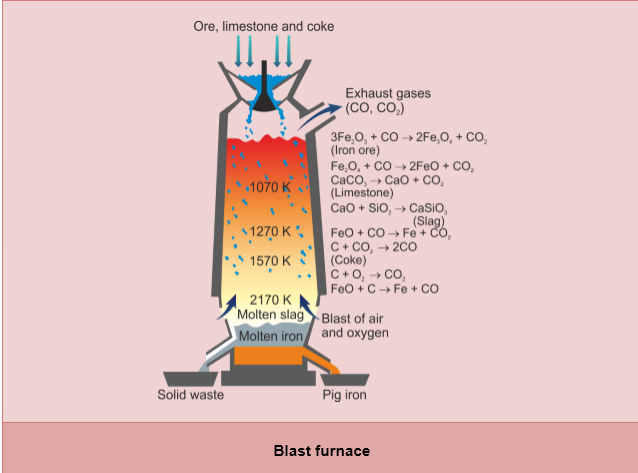
- The reason why we use blast furnace to extract iron is that carbon is more reactive than iron
- The ores of iron are mainly haematite and magnetite
- The ore we usually use in blast furnace is haematite which mainly consists of iron(III) oxide
- Limestone is used to provide carbon dioxide and to react with sand to form slag
Extraction of aluminium
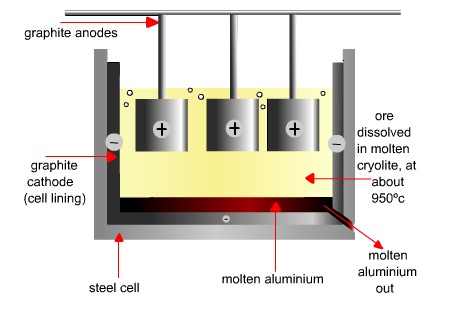
- The reason why we use electrolysis to extract aluminium is that aluminium is more reactive than carbon
- Oxygen will form at anode, thus the carbon anode will be replaced regularly since it will react with oxygen to form carbon dioxide
- Aluminium forms at cathode
- In industrial application, bauxite will be mixed with cryolite to lower its melting point and increase its ability to conduct electricity
Extraction of zinc
- Since the reactivity of zinc is very similar to that of carbon, we can use both electrolysis and carbon to extract zinc
- We roast zinc blende in the air, which will form zinc oxide and sulphur dioxide
- Then, we can use either carbon or carbon monoxide to reduce zinc oxider to form zinc
本博客所有文章除特别声明外,均采用 CC BY-SA 4.0 协议 ,转载请注明出处!